Description
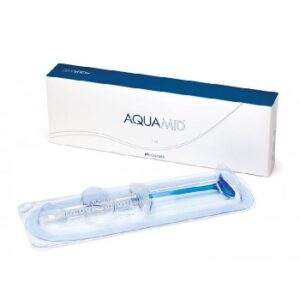
Buy Juvederm Ultra 3 (2x1ml)
JUVEDERM ULTRA 3 is an injectable implant indicated to fill medium and deep skin depressions by injection into the middle dermis and to increase the volume of the lips. Buy Juvederm Ultra 3 (2x1ml)
JUVEDERM ULTRA 3 is a sterile, pyrogen-free and physiological gel of cross-linked hyaluronic acid of non-animal origin.
Biodegradable, viscoelastic, transparent, colorless and homogenized. It consists of cross-linked hyaluronic acid (HA) produced by the bacteria Streptococcus equi, formulated at a concentration of 24 mg/ml and 0.3% of lidocaine in a physiological buffer.
COMPOSITION
- Cross-linked hyaluronic acid 24mg/ ml + Chlorhydrate de lidocaine 0,3%.
- Lidocain hydrochloride 3 mg
- Phosphate buffer pH 7.2 q.s.
- Volumizing capacity : 3/5
- The presence of lidocaine is meant to reduce the patient’s pain during treatment.
PACKAGING
- Conditionnement : 2 x 1 ml
- Needles : 4 x 27 G1/2 (CE 0123)
- One syringe contains 1.0 ml of JUVEDERM ULTRA 3.
- The gel is presented in a graduated, pre-filled, disposable syringe. Each box contains two 1.0mL.
- JUVEDERM ULTRA 3 syringes, 4 single-use 27G1/2” sterile needles to be used only for injecting JUVEDERM ULTRA 3, an instruction leaflet and a set of labels in order to ensure traceability.
INDICATION
JUVEDERM ULTRA 3 is an injectable implant used for filling mid and/or deep depressions of the skin via mid and/or deep dermis injection, as well as for lip definition and enhancement.
AREAS OF INJECTION
- Treats moderate depression to skin deep.
- Ourlement and increase lip volume.
- Moderate nasolabial folds, marionette lines and lip volume.
DURATION
Advanced technology 3D matrix, for greater durability up to 1 year and optimal volumatrice capacity.










Reviews
There are no reviews yet.