Description
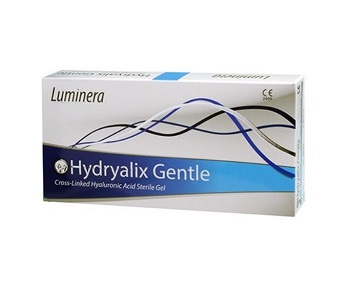
Luminera Hydryalix Gentle (2×1.25ml)
Hydralix Gentle is indicated for superficial lines, fine to moderate wrinkles and minor skin damages. It can be used on wrinkles, lines and creases around the mouth, excellent as a skin booster. Luminera Hydryalix Gentle (2×1.25ml)
Recommended needle 30G.
What is Luminera Hydryalix Gentle (2×1.25ml)?
Luminera’s state of the art technology for the production of Hydryalix, our cross linked HA line of products, gives the advantages of both monophasic cross linking and biphasic gel properties.
Monophasic gel, which is homogenously cross linked, generates a smooth, uniform gel, lowers side effects, such as swelling and the Tyndall effect, and uses less BDDE for the cross linking process. Monophasic gel is 100% cross linked.
Biphasic gel has two phases, a highly cross linked phase of HA particles and a non-cross linked HA phase in which the particles are suspended. This non cross linked phase is needed to enable the injection.
Where can i buy Luminera Hydryalix Gentle (2×1.25ml)?
In our Hybrid MoBiTM technology, the gel is monophasic, fully homogenous , smooth and easy to inject. The gel is then cut to different particle sizes, generating a line of differentiated products which are intended for diverse indications. The particle nature of the gel gives the ability to mold the injected material to the desired shape in the tissue and gives the product its firmness. A property not found in the standard monophasic products which are available in the market.









Reviews
There are no reviews yet.